Purine and Pyrimidine Metabolism
Purines and Pyrimidines
Introduction
Purines and pyrimidines are the two families of nitrogenous bases that make up nucleic acids – in other words, they are the building blocks of DNA and RNA. While they are similar in many respects, there are a number of key differences between them that you will be expected to know for the AP® exam. Before we get into those, however, let’s make sure you understand what purines and pyrimidines are so you can recognize questions about them even if the wording is tricky.
What are Purines and Pyrimidines?: The Basics
Each DNA strand has a ‘backbone’ that is made up of a sugar-phosphate chain. Attached to each one of these sugars is a nitrogenous base that is composed of carbon and nitrogen rings. The number of rings this base has determines whether the base is a purine (two rings) or a pyrimidine (one ring). The purines on one strand of DNA form hydrogen bonds with the corresponding pyrimidines on the opposite strand of DNA, and vice versa, to hold the two strands together. Within DNA molecules, this is their most important function and is known as base pairing. Because hydrogen bonds are not as strong as covalent bonds, base pairings can easily be separated, allowing for replication and transcription.
Because purines always bind with pyrimidines – known as complementary pairing – the ratio of the two will always be constant within a DNA molecule. In other words, one strand of DNA will always be an exact complement of the other as far as purines and pyrimidines go.This phenomenon is known as Chargaff’s Rule, named after Irwin Chargaff, who first noticed it. This complementary pairing occurs because the respective sizes of the bases and because of the kinds of hydrogen bonds that are possible between them (they pair more favorably with bases with which they can have the maximum amount of hydrogen bonds).
There are two main types of purine: Adenine and Guanine. Both of these occur in both DNA and RNA. There are three main types of pyrimidines, however only one of them exists in both DNA and RNA: Cytosine. The other two are Uracil, which is RNA exclusive, and Thymine, which is DNA exclusive. One strategy that may help you remember this is to think of pyrimidines like pyramids that have sharp and pointy tops. So sharp and pointy in fact, that they might CUT (Cytosine, Uracil, Thymine) you.
Which purines pair with which pyrimidines is always constant, as is the number of hydrogen bonds between them:
- ADENINE pairs with THYMINE (A::T) with two hydrogen bonds
- GUANINE pairs with CYTOSINE (G::C) with three hydrogen bonds
One way to remember which bases go together is to look at the shapes of the letters themselves. The letters made up of only straight lines (A and T) are paired with each other, while the letters that are made up of curves (G and C) also go together. Just make sure you don’t write your A’s in cursive!
These specific pairings also factor into Chargaff’s Rule, which we mentioned before. The number of adenines in a DNA molecule will always be equal to the number of thymines. The same goes for guanines and cytosines. Because of this, if you know the percentage of one nitrogen base within a DNA molecule, you can figure out the percentages of each of the other three as well – its complementary pair will have the same percentage, and each of the other two bases will be the sum of the first pair subtracted from 100% and divided by two. Expect a question asking you to calculate something similar to this on the exam.
If what we have covered so far is confusing to you, make sure you go back and review your notes on DNA/RNA structure before moving on to studying the differences between purines and pyrimidines.
Purines vs. Pyrimidines
When it comes identifying the main differences between purines and pyrimidines, what you’ll want to remember is the ‘three S’s’: Structure, Size, and Source. The very basics of what you need to know are in the table below, but you can find more details about each one further down.
Purines | Pyrimidines | |
Structure | Double carbon-nitrogen ring with four nitrogen atoms | Single carbon-nitrogen ring with two nitrogen atoms |
Size | Bigger | Smaller |
Source | Adenine and Guanine in both DNA and RNA | Cytosine in both DNA and RNA Uracil only in RNA Thymine only in DNA |
The most important difference that you will need to know between purines and pyrimidines is how they differ in their structures.
The purines (adenine and guanine) have a two-ringed structure consisting of a nine-membered molecule with four nitrogen atoms, as you can see in the two figures below.
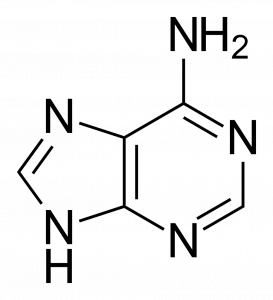
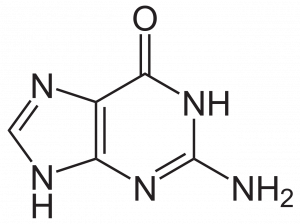
The pyrimidines (cytosine, uracil, and thymine) only have one single ring, which has just six members and two nitrogen atoms.
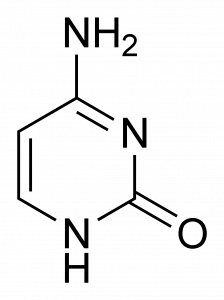
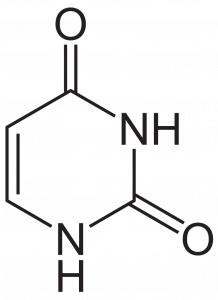
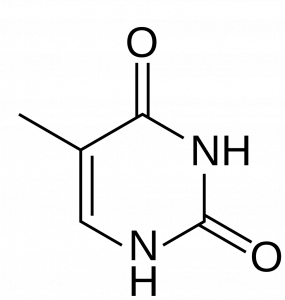
Because purines are essentially pyrimidines fused with a second ring, they are obviously bigger than pyrimidines. This size difference is part of the reason that complementary pairing occurs. If the purines in DNA strands bonded to each other instead of to the pyrimidines, they would be so wide that the pyrimidines would not be able to reach other pyrimidines or purines on the other side! The space between them would be so large that the DNA strand would not be able to be held together. Likewise, if the pyrimidines in DNA bonded together, there would not be enough space for the purines.
Visitor Kindly Note: This application is created solely for the students to view or download an e-books, Competitive Study Notes & other Study materials for free of cost.
Library team try to Helping the students and others who cannot afford buying books is our aim. If you think this Study Material/Book is Useful,
Please Get It Legally from the publishers & If you feel good Share this Application with Others.
Disclaimer: Library does not own this book/materials, neither created nor scanned.
we provide the links which is already available on the internet. For any quarries,
Disclaimer are requested to kindly contact us, We assured you we will do our best.
We DO NOT SUPPORT PIRACY, this copy was provided for students who are financially
troubled but deserving to learn. Thank you
Comments
Post a Comment